IVC Blood Clot Filter Lawyers
Have you or a loved one received a IVC Filter that caused injury? Call us now if you need assistance with your legal representation.
IVC Blood Clot Filter Lawyers
Have you or a loved one received a IVC Filter that caused injury? Call us now if you need assistance with your legal representation.
IVC Filters: History, Purpose, Dangerous Failures, Government Sanctions and Lawsuits
What is an IVC Filter?
IVC filters prevent a blood clot (deep vein thrombosis) in the legs or pelvis from reaching the lungs where it can cause a fatal pulmonary embolism.
IVC filters are small, metal “cage-like” devices, (sometimes described as “spiders”) designed to trap clots in their long thin metal legs. They are inserted in the inferior vena cava (IVC) near the kidneys. The inferior vena cava is the largest vein in the body. It returns deoxygenated blood from the legs and organs to the lungs where the blood is infused with oxygen. The blood then moves into the heart and is circulated trapped in the filter until the body can dissolve it, sometimes with the aid of anti-coagulants (blood thinners).
Dangerous IVC Failures
If you have an IVC blood clot filter failure you may need Wayne Wright LLP. These devices can cause serious injuries and you need a law firm with the expertise and resources to fight for everything you deserve.
Blood clots can escape from the IVC filter. If it tilts, the clot can flow past it. If pieces of the filters legs break due to metal fatigue or twist, the clot can escape. It can then move into the lungs, causing a potentially fatal pulmonary embolism. The IVC filter can also “migrate” to another part of the vein, perhaps reducing its ability to trap clots. Once it has moved out of position, its legs can pierce part of the vein. If pieces of the filter break off, they can spear the heart, the lungs and the vein.
FDA Warnings about IVC filters
In 2010, the U.S. Food and Drug Administration (FDA) became alarmed at rate the IVC filters were failing. A 2010 FDA safety alert listed 921 serious and potentially deadly IVC filter failures: 328 cases of IVC migration, 146 cases of embolization (instances in which part of the device broke loose), 70 cases in which the IVC perforated the vein and 56 cases of filter fracture. There are considerable risks if retrievable IVC devices are left in place too long. They include blood vessel damage, bruising, bleeding at the implantation site, damage to nearby organs and filter detachment, migration and fracture resulting in potentially fatal injuries to the heart and lungs.
Prosecutions of IVC manufactures for fraud
Lawsuits have been filed against C.R. Bard and Cook Group, the manufacturer of the Bard® Recovery™ filter, the Bard G2® filter and the Bard G2X® Express filter. The long metal legs of these filters have broken off and pierced the heart, blood vessels and major organs – all potentially life threatening events. The filters themselves have detached from the implantation site and migrated to other parts of the body.
Bard has been charged with knowingly selling IVC filters with design flaws and manufacturing defects. The FDA says the company illegally used a medical tool to remove IVC filters that it had not approved and did not report it. Because of these issues, the FDA is urging doctors to remove retrievable IVC filters as soon as the danger of a clot has passed.
In 1993, C. R. Bard Inc. pleaded guilty to a “391 count criminal indictment and was fined $61 million” after it admitting “it had illegally experimented on patients, lied to the government about the experiments and the devices’ failures and sold catheters without approval” of the FDA. Six company officials were charged with a conspiracy that caused multiple “emergencies and one death,” according to a 1995 article in The New York Times.
Why Doctors Prescribe IVC Filters
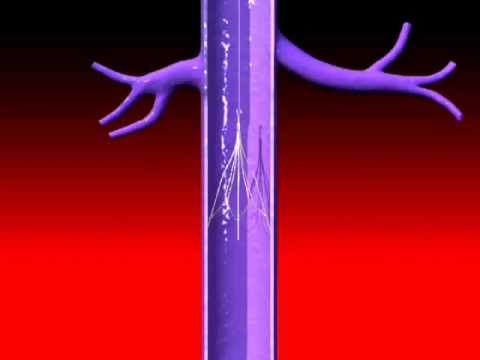 When life threatening blood clots develop in the legs or the pelvis, anti-coagulants (blood thinners) are a physician’s first choice to dissolve them. In some cases they may not be able to prescribe them. They may interfere with a patient’s other medications. The patient’s blood clots may have recurred after taking anti-coagulants. The patient may have had a recent surgery, trauma, or side effect that rules out the use of blood thinners. An IVC filter may then be implanted. The procedure is performed by an “interventional radiologist, interventional cardiologist or a vascular surgeon.”
When life threatening blood clots develop in the legs or the pelvis, anti-coagulants (blood thinners) are a physician’s first choice to dissolve them. In some cases they may not be able to prescribe them. They may interfere with a patient’s other medications. The patient’s blood clots may have recurred after taking anti-coagulants. The patient may have had a recent surgery, trauma, or side effect that rules out the use of blood thinners. An IVC filter may then be implanted. The procedure is performed by an “interventional radiologist, interventional cardiologist or a vascular surgeon.”
History of IVC Filters
IVC filters have been in use for nearly 50 years. The first “…open surgical placement of a filter” occurred in 1967 and the “first percutaneous insertion of a filter took place in 1984.” According to the FDA, their usage has rapidly increased. In 1979, 2,000 IVC filters were implanted in patients with threatening blood clots. In 2007, 167,000 IVC filters were implanted. By 2012, the number had grown to 260,000 with the anticipation that it would continue increasing.
Because of the serious malfunctions of some IVC filters, the FDA to issue strict warnings to physicians, urging careful monitoring of the filters and removal as soon as possible to avoid complications.
Who is at Risk for Blood Clots?
Orthopedic surgeries that reduce blood flow exponentially increase the risk of blood clots (deep vein thrombosis). The chances of a clot are high “during or soon after hip or knee replacement surgery.” The U.S. National Library of Medicine reports that two kinds of clots can develop after these operations: a deep vein thrombosis (DVT) a blood clots in the leg and a pulmonary embolism, a blood clot in the lungs (PE).
Knee replacement surgery and hip replacement surgery can release tissue debris, protein and fats into a vein, damaging it. When veins are damaged, they release blood clotting substances. Blood clots can also occur after a broken hip or leg, cancer treatment, a previous history of blood clots, spinal cord paralysis, post-menopausal hormone replacement therapy, varicose veins, pregnancy, natural childbirth or a C-section, a history of heart attack, stroke or congestive heart failure and inflammatory bowel disease.
If you feel that your IVC filter is putting you or a loved one at risk, don’t hesitate to contact our lawyers for a free consultation to evaluate your situation.
IVC Filters: History, Purpose, Dangerous Failures, Government Sanctions and Lawsuits
What is an IVC Filter?
IVC filters prevent a blood clot (deep vein thrombosis) in the legs or pelvis from reaching the lungs where it can cause a fatal pulmonary embolism.
IVC filters are small, metal “cage-like” devices, (sometimes described as “spiders”) designed to trap clots in their long thin metal legs. They are inserted in the inferior vena cava (IVC) near the kidneys. The inferior vena cava is the largest vein in the body. It returns deoxygenated blood from the legs and organs to the lungs where the blood is infused with oxygen. The blood then moves into the heart and is circulated trapped in the filter until the body can dissolve it, sometimes with the aid of anti-coagulants (blood thinners).
Dangerous IVC Failures
If you have an IVC blood clot filter failure you may need Wayne Wright LLP. These devices can cause serious injuries and you need a law firm with the expertise and resources to fight for everything you deserve.
Blood clots can escape from the IVC filter. If it tilts, the clot can flow past it. If pieces of the filters legs break due to metal fatigue or twist, the clot can escape. It can then move into the lungs, causing a potentially fatal pulmonary embolism. The IVC filter can also “migrate” to another part of the vein, perhaps reducing its ability to trap clots. Once it has moved out of position, its legs can pierce part of the vein. If pieces of the filter break off, they can spear the heart, the lungs and the vein.
FDA Warnings about IVC filters
In 2010, the U.S. Food and Drug Administration (FDA) became alarmed at rate the IVC filters were failing. A 2010 FDA safety alert listed 921 serious and potentially deadly IVC filter failures: 328 cases of IVC migration, 146 cases of embolization (instances in which part of the device broke loose), 70 cases in which the IVC perforated the vein and 56 cases of filter fracture. There are considerable risks if retrievable IVC devices are left in place too long. They include blood vessel damage, bruising, bleeding at the implantation site, damage to nearby organs and filter detachment, migration and fracture resulting in potentially fatal injuries to the heart and lungs.
Prosecutions of IVC manufactures for fraud
Lawsuits have been filed against C.R. Bard and Cook Group, the manufacturer of the Bard® Recovery™ filter, the Bard G2® filter and the Bard G2X® Express filter. The long metal legs of these filters have broken off and pierced the heart, blood vessels and major organs – all potentially life threatening events. The filters themselves have detached from the implantation site and migrated to other parts of the body.
Bard has been charged with knowingly selling IVC filters with design flaws and manufacturing defects. The FDA says the company illegally used a medical tool to remove IVC filters that it had not approved and did not report it. Because of these issues, the FDA is urging doctors to remove retrievable IVC filters as soon as the danger of a clot has passed.
In 1993, C. R. Bard Inc. pleaded guilty to a “391 count criminal indictment and was fined $61 million” after it admitting “it had illegally experimented on patients, lied to the government about the experiments and the devices’ failures and sold catheters without approval” of the FDA. Six company officials were charged with a conspiracy that caused multiple “emergencies and one death,” according to a 1995 article in The New York Times.
Why Doctors Prescribe IVC Filters
When life threatening blood clots develop in the legs or the pelvis, anti-coagulants (blood thinners) are a physician’s first choice to dissolve them. In some cases they may not be able to prescribe them. They may interfere with a patient’s other medications. The patient’s blood clots may have recurred after taking anti-coagulants. The patient may have had a recent surgery, trauma, or side effect that rules out the use of blood thinners. An IVC filter may then be implanted. The procedure is performed by an “interventional radiologist, interventional cardiologist or a vascular surgeon.”
History of IVC Filters
IVC filters have been in use for nearly 50 years. The first “…open surgical placement of a filter” occurred in 1967 and the “first percutaneous insertion of a filter took place in 1984.” According to the FDA, their usage has rapidly increased. In 1979, 2,000 IVC filters were implanted in patients with threatening blood clots. In 2007, 167,000 IVC filters were implanted. By 2012, the number had grown to 260,000 with the anticipation that it would continue increasing.
Because of the serious malfunctions of some IVC filters, the FDA to issue strict warnings to physicians, urging careful monitoring of the filters and removal as soon as possible to avoid complications.
Who is at Risk for Blood Clots?
Orthopedic surgeries that reduce blood flow exponentially increase the risk of blood clots (deep vein thrombosis). The chances of a clot are high “during or soon after hip or knee replacement surgery.” The U.S. National Library of Medicine reports that two kinds of clots can develop after these operations: a deep vein thrombosis (DVT) a blood clots in the leg and a pulmonary embolism, a blood clot in the lungs (PE).
Knee replacement surgery and hip replacement surgery can release tissue debris, protein and fats into a vein, damaging it. When veins are damaged, they release blood clotting substances. Blood clots can also occur after a broken hip or leg, cancer treatment, a previous history of blood clots, spinal cord paralysis, post-menopausal hormone replacement therapy, varicose veins, pregnancy, natural childbirth or a C-section, a history of heart attack, stroke or congestive heart failure and inflammatory bowel disease.
If you feel that your IVC filter is putting you or a loved one at risk, don’t hesitate to contact our lawyers for a free consultation to evaluate your situation.
